Which Describes the Current Model of the Atom
Describe the current model of the atom. A solid sphere unique for everything that exists.

The Development Of The Atomic Model Wired
Ernest rutherford performed a famous.

. An atom is mostly empty space with a small dense positively charged center. An unknown element has two primary isotopes one with a relative abundance of 2534 percent and a mass of 5235 u and another with a relative abundance of 7466 percent and a mass of 5487 u. API When visualizing the atom which atomic model provides a better picture.
C- it is similar to the model of plum pudding with suspended plums. It is only accepted by a small fraction of scientists. Select all that apply Multiple select question.
Who are the experts. 2 points It can be used to predict atomic behavior in most circumstances. Which best describes the current model of an atom.
B-it has remained the same for several centuries. The electrons make up the nucleus of the atom. It has been perfected and will never be changed in the future.
It has remained the same for several centuries. Which of the following statements best describes the current model of the atom. Chemistry 09082019 2120 GravityShifter13.
A central nucleus with proton neutrons and electrons orbiting in levels of high probability. The current model of the atom shows that the atom is mainly empty space. Due to this high probability orbits are replaced by the term orbital.
It is only useful in very specific circumstances. View the full answer. Which best describes how the current scientific model of the atom was developed.
Which describes the current model of the atom. The number of neutrons in the nucleus equals the number of protons. The current model of atomic theory is called the Quantum Mechanical Model otherwise known as the Electron Cloud Model.
Which best describes the current model of the atom. Germanium 403 Label the atomic symbol for the element. It has been perfected and will never be changed in the future.
This current atomic model evolved from the earlier Rutherford-Bohr model which compared electrons orbiting an atomic nucleus to planets orbiting the sun. Which best describes the current model of the atom. Which best explains why scientific theories grow stronger over time.
A-it is similar to the model of the solar system with orbiting planets. It is similar to the model of plum pudding with suspended plums. A central nucleus containing protons and neutrons with electrons orbiting in levels of high probability.
We review their content and use your feedback to keep the quality high. There is a small part of the atom called the nucleus which contains most of the mass of the atom and all of its positive. Which describes the current model of the atom.
Which describes the current model of the atom. Jan 9 2017. Experts are tested by Chegg as specialists in their subject area.
According to the new current Quantum atomic model An orbital is a region of space in an atom where the. A solid sphere with electrons and protons embedded. The model was the result of hundreds of years of experiments.
Dt Draw a Lewis diagram for each element. D- it may be revised with increasing scientific knowledge. The mass of the atom is mostly the mass of the neutrons and protons.
It is similar to the model of the solar system with orbiting planets. Which best describes the current model of the atom. Which best describes the current model of an atom.
It is only useful in very specific circumstances. The current model of atom describes the presence of a centr. Oh okay In the atomic orbital model the atom consists of a nucleus surrounded by orbiting electrons.
It can be used to predict atomic behavior in most circumstances. Which of the following correctly describe the current model of the atom. Which best describes the current model of the atom.
An atom consists of a central nucleus with proton neutrons and electrons orbiting in levels of high probability. These electrons exist in atomic orbitals which are a set of quantum states of the negatively charged electrons trapped in the electrical field generated by the positively charged nucleus. The electrons are located outside of the the nucleus but the most we can know about their location is the volume of space in which they can be found 90 of the time.
The protons and neutrons are found in the nucleus the small center of the atom with hardly any mass. It can be used to predict atomic behavior in most circumstances. According to the Bohrs atomic model the electrons revolve around the nucleus in a fixed orbit.
The newest understanding of atomic makeup in the Electron Cloud Model better. It is only accepted by a small fraction of scientists. This was based upon the bright-line spectra of molecular hydrogen and lead to postulates that describe the electronic structure of the atom as having electrons in discrete energy levels and orbiting the nucleus much like planets orbit a star.
The solar system model of the atom describes a situation in which the atom would collapse. Classically the orbits can be likened to the planets orbiting the sun. It has been perfected and will never be changed in the future.

Atomic Structure Essential Question Describe How The Model Of The Atom Has Changed Since The Greek Idea Of Atomos Ppt Video Online Download

Development Of The Atomic Theory What You Should Be Able To Do After Studying This Lesson Chapter 1 Describe Some Of T Atomic Theory 8th Grade Science Chapter
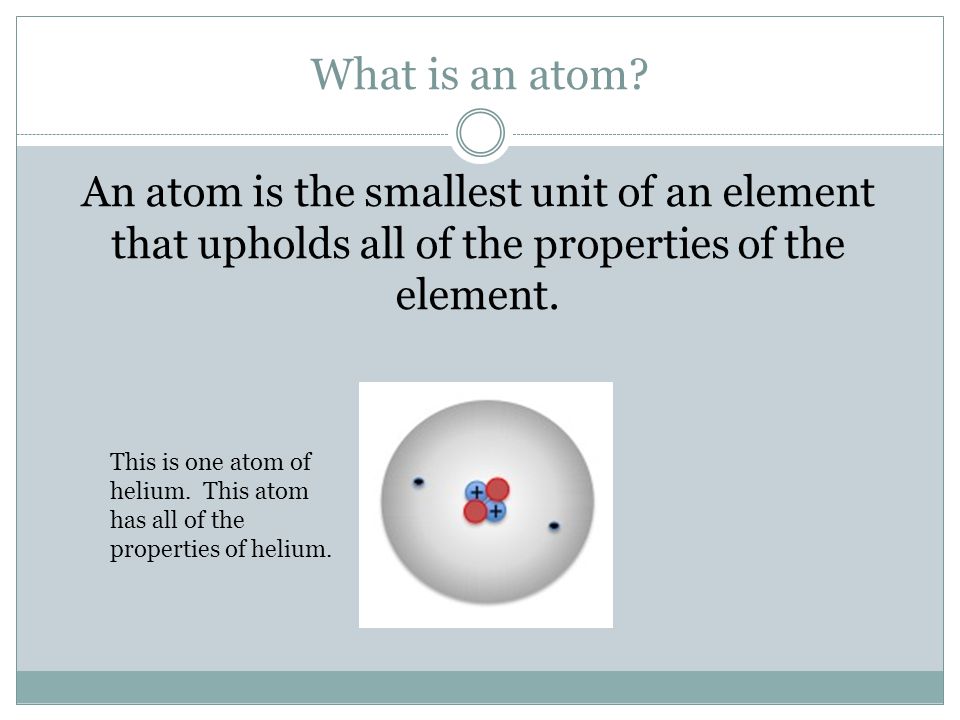
Atoms 8 5a The Student Is Expected To Describe The Structure Of Atoms Including The Masses Electrical Charges And Locations Of Protons And Neutrons Ppt Video Online Download
0 Response to "Which Describes the Current Model of the Atom"
Post a Comment